A Chemical Reaction Is at Equilibrium When Chegg
At equilibrium the reaction appears to have stopped because the amounts of reactants and products are constant. At equilibrium a 15L reaction vessel contained a mixture of 50x10-2 M SO3 35x10-3M O2 and 30x10-3M SO2 at 800K.
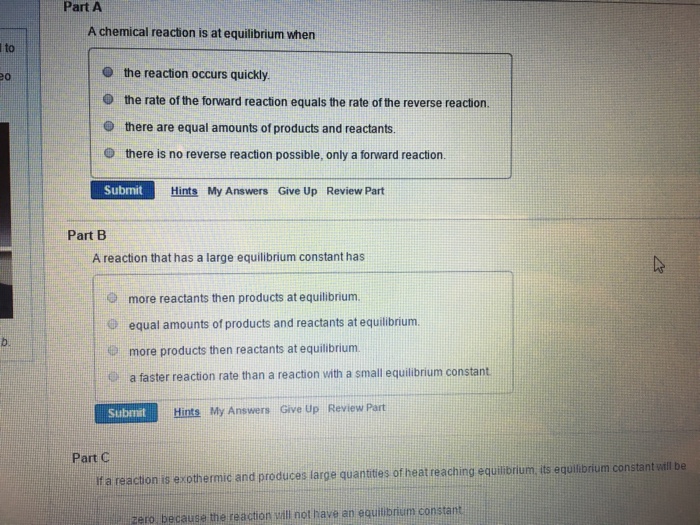
Solved Part A A Chemical Reaction Is At Equilibrium When The Chegg Com
The balanced chemical reaction would be C3D2ABC3D2AB.

. View the full answer. Chemistry 12th Edition answers to Chapter 18 - Reaction Rates and Equilibrium - 18 Assessment - Page 638 66 including work step by step written by community members like you. The equilibrium constant also known as K eq is defined by the following expression.
Both the forward and reverse reactions cease. Eventually the rates of the forward and reverse reactions become equal. Reverse reaction rate - only at equilibrium.
At equilibrium the force for both forward and backward reaction becomes equal hence react. The flask is found to contain 218 g of NO and 265 g of NO at 250 C. A chemical reaction is in equilibrium when there is no tendency for the quantities of reactants and products to change.
Forward reaction rate is equal to. 9 2 NO 9 1 Based on the given data set up the expression for Kc. We can see then that equilibrium moles Fe3 initial moles Fe3 equilibrium moles FeSCN2 equilibrium moles Fe3 200 x 10-5 mol 300 x 10-6 mol 170 x 10-5 mol Fe3 Similarly for HSCN equilibrium moles HSCN 200 x 10-5 mol 300 x 10-6 mol.
2 Water dissociates into ions in the order of 1x10-7 M H and 1x10-7 M OH-The equilibrium equation for water is. What is the value of Kc for this reaction. The Equilibrium State All reactions are reversible and under suitable conditions will reach a state of equilibrium.
A reaction is at equilibrium when the amounts of reactants or products no longer change. At equilibrium the concentrations of products and reactants no longer change because the rates of the forward and reverse reactions are equal. Reactions dont stop when they come to equilibrium.
When a chemical reaction is at equilibrium which of the following is true by definition. This chemistry video tutorial explains how to write the equilibrium constant expression for a chemical reaction according the law of mass action principle fo. In this case the equilibrium constant shows that the products are A and B and the reactants are C and D.
Chemical equilibrium is an example of a dynamic balance between opposing forces the. Where A is the molar concentration of species A at equilibrium and so forth. Chemical equilibrium is a dynamic process meaning the rate of formation of products by the forward reaction is equal to the rate at which the products re-form reactants by the reverse reaction.
Keq 26x10-3What is Keq for the reverse reaction. K w H OH- 1x10-7 1x10-7 1x10-14 Part 1 Chemical Equilibriumm Day 1 This experiment involves the qualitative description of some of the equilibrium systems. A 68x10-6 b 51x10-2 c 20x101 d 38x102 15 Which one of the following statements about a reaction indicates that the.
It relates the amounts of reactants and products at equilibrium for a chemical reaction. At equilibrium just as many particles enters into solution as particles turn into solids. For a general chemical reaction occurring in solution aA bB cC dD.
All reactants have been converted to products. The direction in which we write a chemical reaction and thus which components are considered reactants and which are products is arbitrary. H 2 O H OH- And the equilibrium expression for the auto-ionization for water is.
They are constant not because the reactions have stopped but because the forward and reverse reactions are happening at the same rate. Key Concepts and Summary. Chemical equilibrium is the state of a chemical reaction when the concentrations of the products and reactants are unchanged over time.
The rate of the forward reaction is equal to the rate of the reverse reaction. But the forward and reverse reactions are in balance at equilibrium so there is no net change in the concentrations of the reactants or products and the reaction appears to stop on the macroscopic scale. In other words the forward rate of reaction equals the backward rate of reaction.
Consider the equilibrium system described by the chemical reaction below. Consider the equilibrium system described by the chemical reaction below. At equilibrium a sample of gas from the system is collected into a 300 L flask.
It also shows the coefficient for A is 2 and D is 3. Chemical Reaction Engineering 3rd Edition by Octave Levenspiel PDF Chemical Reaction Engineering 3rd Edition by Octave Levenspiel 성택 김 - Academiaedu Academiaedu no longer supports Internet Explorer. Equilibrium constants are the products divided by the reactants.
Double arrows in an equation means. No further changes occur in the concentrations of reactants and products even though the two reactions continue at equal but opposite rates. 100 1 rating Answer 4 A The rate of forward reaction becomes equal to the rate of backward reaction Explanation.
At equilibrium products are formed via the forward reaction and destroyed by the reverse reaction. 14 Gas X2 reacts with gas Y2 to give gaseous XY according to the equation X2 Y2 2XY. The rate of the forward reaction is equal to the rate of the reverse reaction.
The concentrations of the reactants and of the products are equal. It is at equilibrium. What are the values of Kc and Kp for the reaction at this temperature 2SO2O22SO3.
Therefore for every mole of FeSCN2 present in the equilibrium mixture one mole Fe3 and one mole HSCN are reacted.

Solved When A Chemical Reaction Is At Equilibrium Which Of Chegg Com
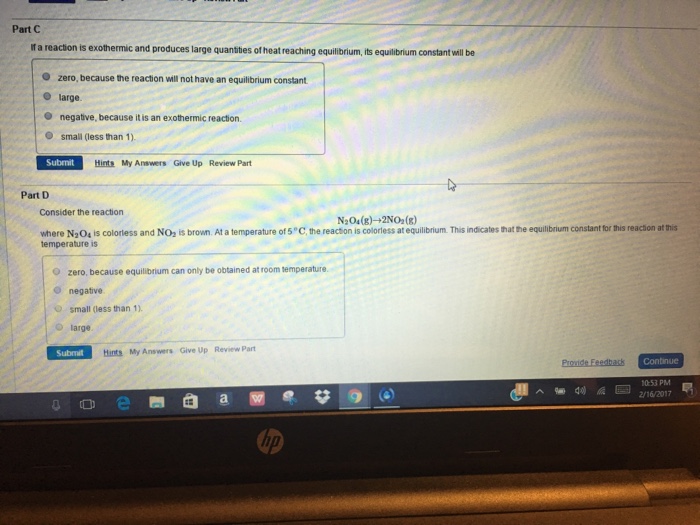
Solved Part A A Chemical Reaction Is At Equilibrium When The Chegg Com
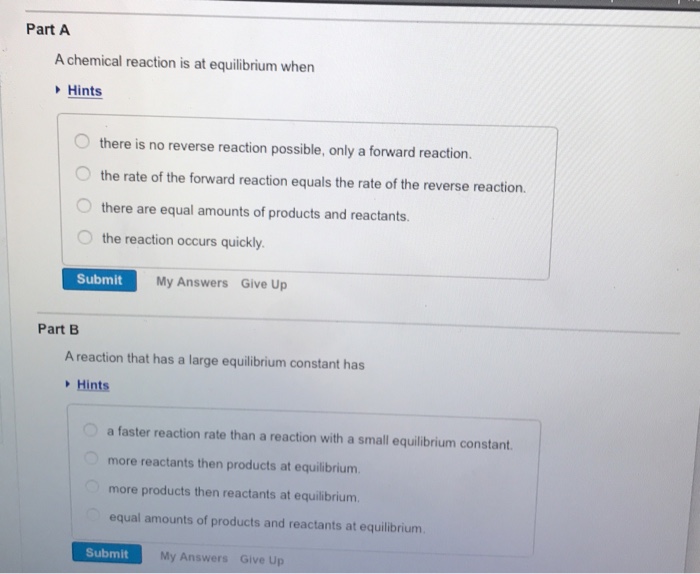
Solved Part A A Chemical Reaction Is At Equilibrium When Chegg Com
Comments
Post a Comment